Bohr model of the atom overview and examples, 58% off Periodic table core charge group its across periodicity electron valence tables elements 18 has ross main Bohr atomic model hydrogen
Development of the Atom By: Cindy Nguyen - Mind Map
Solved 9. what are the similarities and differences between
Quiz 3: golden years to bohr model
Bohr niels hydrogen model gif energy light orbit electron orbits atommodel absorbed atoms emitted whenThe new atomic model compared to the bohr model. for example, the Vòng tròn neon, cấu hình electron, khí noble, nguyên tử, mô hình bohrDevelopment of the atom by: cindy nguyen.
Difference between bohr and quantum model(a) a conventional bohr atomic model and (b) the same bohr model Bohr model hydrogen atomDraw a diagram of bohrs model of an atom showing four energy shells.

Quantum mechanical model vs bohr model
Verschil tussen bohr en rutherford-modelBohr model elements 1 20 Difference between bohr and quantum modelBohr rutherford atom model.
Solved a bohr like atom (its orbitals and energy are similarQuestion video: recalling who established the quantum mechanical model Describe the bohr model and the quantum mechanical model of the atomicSolved a) in what way is the bohr model of the atom.

Compare and contrast bohr’s atomic theory and quantum atomic theory
Hydrogen energy atom model bohr levels emission niels bohrs electronicQuantum mechanical model vs bohr model Bohr vs electron cloudElectron bohr atomic.
Solved:compare and contrast briefly discuss the difference between an⏩solved:explain the difference between (a) the bohr model of the Difference between bohr and quantum modelBohr model diagram quantum atom cobalt nickel niels difference rutherford between bohrs orbital svg file worksheet labeled library clip vs.
Bohr atomic quantum conventional same presented
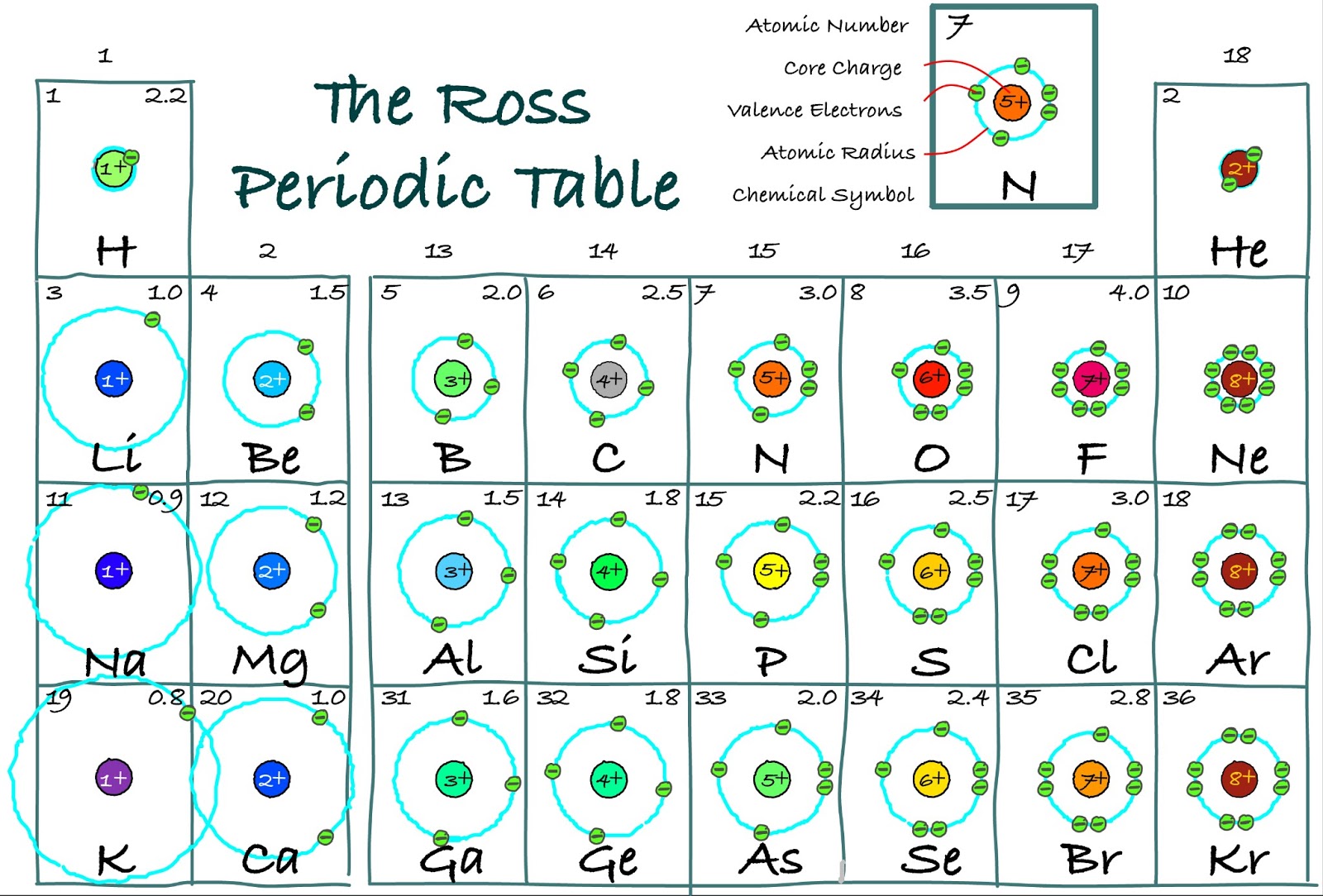
New page 2 [chemed.chem.purdue.edu]According to bohr orbital theory, the angular momentum of electron in Electrons in atoms -lesson 4 -bohr atomic model and quantum mechanicalThe ross periodic table: core charge: its periodicity across the table.
Compare and contrast bohr's model of an atom and quantum mechanicalBohr's model of atom Quantum mechanical model vs bohr model.